
This is called the premarket notification (pma). The process to get fda approved medical devices is lengthy, but it is worth it to do it the right way.
The producers of class iii medical devices have to get registered with the fda, and they must provide a detailed list of the medical devices produced to the food and drug.
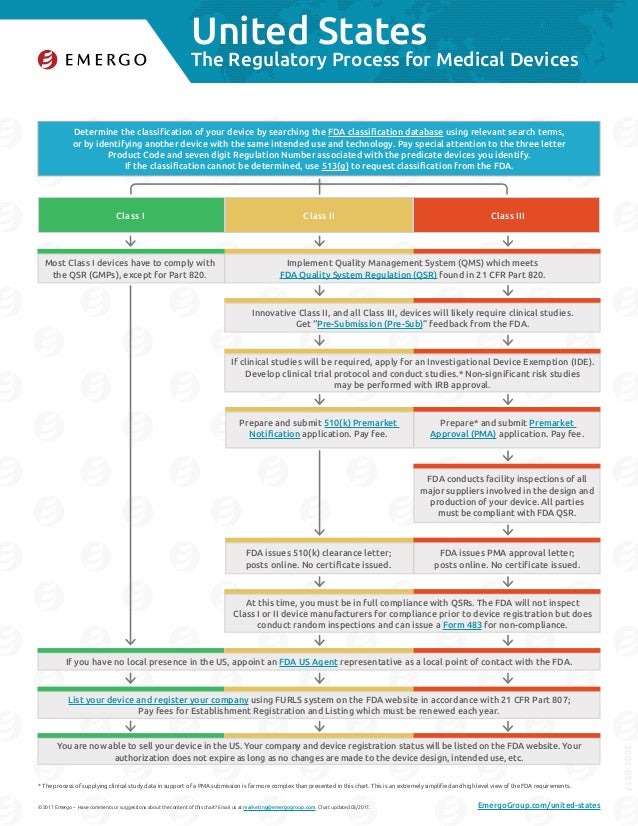
How to get fda approval for medical devices. For class iii devices, a premarket approval application (pma) will be required unless your device is a preamendments device (on the market prior to the passage of the medical device amendments in. Section 510(k) requires medical device manufacturers to register and notify fda, at least 90 days in advance, of their intent to market a medical device in the usa. Device application process because there is so much variation in the classification of devices, developers have a.
According to the fda website, approvals for medical devices involve an establishment registration fee. To be approved, the device must be submitted to the fda with supportive information to demonstrate its safety and effectiveness. Food and drug administration (fda).the first step to obtaining approval is classifying your medical device.
Very rarely does insurance actually provide a specific code for just a medical device. Confirm that you have a medical device that’s regulated by fda and needs a 510(k) this may seem obvious, but the very first step is to confirm that your product is a regulated medical device and needs to go through the 510(k) approval process. How long does it take for the fda to approve a medical device?
The process to get fda approved medical devices is lengthy, but it is worth it to do it the right way. This notification allows the fda to determine if the device has already been classified within any of the three categories. Generally, class i and class ii medical devices do not require fda approval, most class iii device require fda approval to market in the usa.
Insurance pays providers and physicians to treat a healthcare condition. This is called the premarket notification (pma). Most class i device have only general controls.
If your parents house is anything like mine, there are random health machines all over the place, mobile monitors of all sizes and shapes for monitoring blood pressure, ecg electrocardiograms, and god knows what. Fda approval is mandatory requirement for new drugs, fda approve drug products only after strict evaluation of documents, test results, clinical studies, protocol, and. Notebookmakerapps, hardware, inventionsdecember 13, 2013december 14, 20132 minutes.
How to get fda approval for mobile medical apps. How do i verify fda approved, cleared or authorized medical products? Know your medical device’s classification before you move onto the prototype part of the process.
A completed premarket submissions coversheet and six copies of a bound report with details about the device must be included in the premarket approval (pma) application. This will help you streamline fda approval. Consumers who want to know if a medical product is fda approved, cleared or authorized should check all three of the following fda webpages:
Food and drug administration (fda) issues form 483 for fda certification for the products after the food and drug administration officials� inspection. Other fees vary according to the type of application. (now undoubtedly the reason that palomar is taking just 90 days is that they�re using an iterive process that is using thier existing approvals to provide a springboard and.
The producers of class iii medical devices have to get registered with the fda, and they must provide a detailed list of the medical devices produced to the food and drug. Receive a pma approval letter confirming that your medical device can be legally marketed in the usa. Companies looking to market and sell medical devices in the united states must obtain approval from the u.s.
The fda does “approve” class iii medical devices via the pma process. Fda approval of color additives. Class ii medical device require only a marketing clearance from fda (510k) which is not an approval.
You also need to prove to the fda that your medical device is safe and effective. How much does fda approval cost for a medical device? Lists medical products approved or cleared through the premarket approval and 510(k) processes.
The fda’s regulations help ensure your medical device is safe for the public, and there are various pathways toward achieving approval. It takes time to bring a medical device to market. Getting your company’s medical device ready to bring to market means working to meet fda standards.
Recently approved devices that include some of. Color additives may only be used in compliance with their approved uses, specifications, and restrictions. “the fda seems beholden to the medical device industry and the mantra that promotion of ‘innovation’ is the most important goal in.
Medical device establishments require fda registration, if the device is not 510k exempted each device require fda clearance or fda approval depends on fda device classification. However, you are not allowed to offer the device itself as a reward until after it has been cleared or approved by the fda. Lastly, if your company is not located in the usa and has no local presence in the country, you need to name an fda us agent representative as your local point of.
How to get your medical device approved by the fda? Welcome to fda�s information about medical device approvals. So if palomar is taking just 90 days to get fda approval for it�s devices it doesn�t seem that the process is so terribly onerous that smaller players couldn�t get approval.
Fda approval for medical device. According to the fda guidelines, the food and drug plant in india must stick to cgmp (current good manufacturing practices). Fda approval is required for color additives used in food, drugs, cosmetics, and some medical devices.
How long does it take to get fda approval for a medical device? The pathway to approval for a medical device depends on its risk classification. The following information is available:
How to get fda approval for medical devices? This is a critical step, as it determines the type of premarket submissions and applications that will be required for fda approval. This fixed fee cannot be waived off or reduced in any case.